Activity 2: Atom and
Atomic Structure
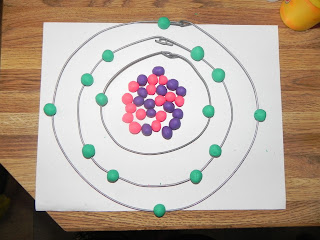
Model 1: Silicon
This is a model of the element Silicon (Si) made out of wire
and play dough. There are 14 purple balls of play dough that represent the
neutrons and 14 pink balls that represent the protons in the nucleus. For this
element there are 14 electrons that are placed on the three levels of wire. There
are 2 on the first level, 8 on the second level and 4 on the third level.
Model 2: Beryllium
This is a model of the element Beryllium (Be) also made out
of wire and play dough. There are 4 pink balls of play dough that represent the
protons and 5 green balls of play dough that represent the neutrons in the nucleus.
For this element there are 4 electrons that are placed on the two levels of
wire. There are 2 on the first level and 2 on the second level.
Model 3: Carbon
This is a model of the element Carbon (C) made out of wire
and play dough as well. There are 6 purple balls of play dough that represent
the neutrons and 6 green balls that represent the protons in the nucleus. For
this element there are 6 electrons that are placed on the two levels of wire.
There are 2 on the first level and 4 on the second level
Questions:
1. What is the atomic number for each of the
models?
o
Silicon: 14
o
Beryllium: 4
o
Carbon: 6
2. What is the atomic mass number for each of the
models?
o
Silicon: 28.0855
o
Beryllium: 9.012182
o
Carbon: 12.0107
3. In the models, which two subatomic particles are
equal in number?
o
The number of protons are equal to the number of
electrons. In the elements that I chose Silicon has 14 protons and 14
electrons, Beryllium has 4 protons and 4 electrons, and Carbon has 6 protons
and 6 electrons.
4. How would you make an isotope for one of your
models? What would change with the model?
o
Silicon has 14 protons and 14 neutrons and the atomic
mass is 28. If I added 1 more neutron to my Silicon model the atomic weight
would be 29.
5. Considering the overall volume of the element
models, what makes up most of the volume of an atom?
o
What makes up most of the volume of an atom is
empty space where as the nucleus that is protons and neutrons make up most of
the mass of the element.
6. For one the models show with another image what
happens when energy excites an electron.
o
When Oxygen gets excited.
7. Once the election is excited, what do we
typically observe when the electron returns to the ground-state?
o
An atom that is excited eventually produces a
quantum of energy, which may be observed as light.
8. Why are some elements different colors when they
are excited?
o
According to our book an atom that is excited produces
a quantum of energy as the electron jumps back down to one of the lower levels
and reaches the ground-state. The color that is produced depends on the amount
of energy that is released.
9. With Fourth of July coming up quickly, explain
how the colors of fireworks arise.
o
The colors of fireworks arise from the different
energy levels of each of the elements. Sodium salts give a yellow flame,
potassium a lavender flame, and lithium is a red flame.
10. Explain the overall organizational structure of
the periodic table.
o
The overall organizational structure of the
periodic table has to do with the vertical columns which are groups that
contain elements with similar chemical properties. As for the horizontal rows which are elements
that demonstrate a range of properties from metallic to nonmetallic.
11. List two example elements for each of these
groups or classes:
o
Alkali Metals: Lithium and Sodium
o
Alkaline Earth: Beryllium and Magnesium
o
Halogens: Fluorine and Chlorine
o
Noble Gases: Helium and Neon
o
Transition Metals: Scandium and Titanium
o
Non-Metals: Hydrogen and Carbon
o
Metalloids: Boron and Silicon



Your models are great! I love the colors and they are very crisp and easy to see and understand. Also I liked how you explained the different components of each and what they were representing underneath the photos. That made me really understand what your models were showing, great job!
ReplyDeleteThere is the playdough again- great! I need to invest in some. Your models are very neat. Your whole blog post is very neat, actually. I do not feel lost, everything is there step by step which makes for great reading.
ReplyDelete